COVID-19 Outpatient Therapeutics Clinical Decision Aid
February 2024
Download as a 543KB PDF
Clinical Decision Aid for Ages 12+ Years
Adult or pediatric patients (ages 12 and older* weighing at least 40 kg) with mild to moderate COVID-19 and at high risk for progression to severe disease.
* Age requirement does not apply for Veklury (remdesivir)
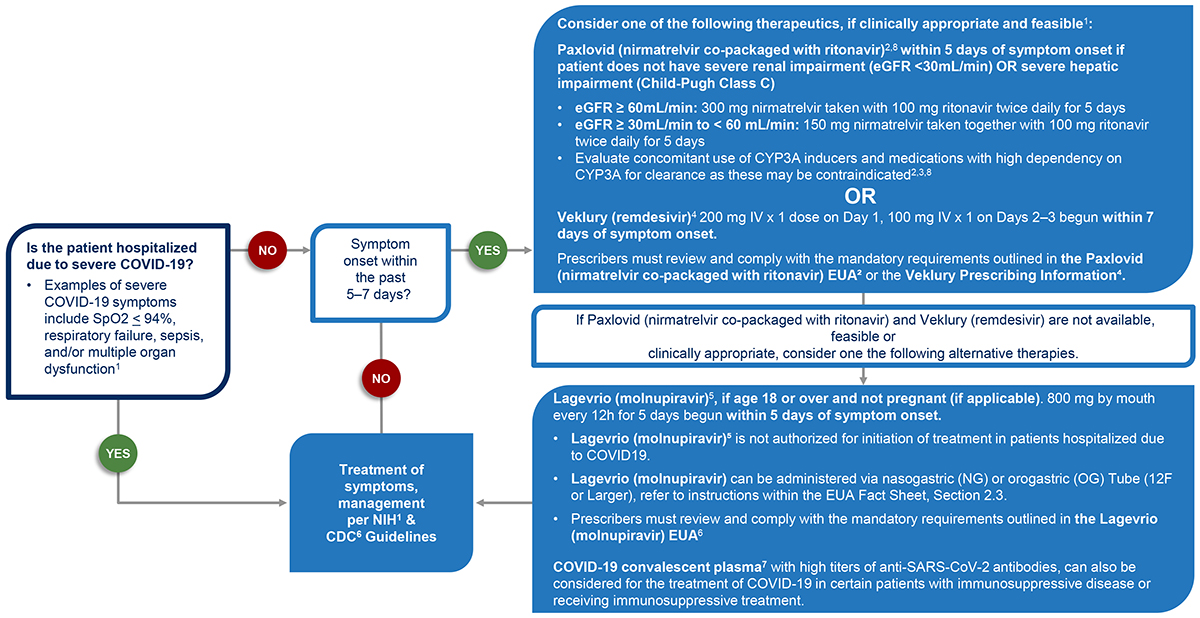
Text Version of Clinical Decision Aid for Ages 12+ Years Flow Chart
Step 1
Is the patient hospitalized due to severe COVID-19?
Examples of severe COVID-19 symptoms include SpO2 ≤ 94%, respiratory failure, sepsis, and/or multiple organ dysfunction.1
Step 2
Symptom onset within the past 5–7 days?
Step 3
Consider one of the following therapeutics:
- If Paxlovid (nirmatrelvir co-packaged with ritonavir) and Veklury (remdesivir) are clinically appropriate and feasible1, continue to
Step 4.
- If Paxlovid (nirmatrelvir co-packaged with ritonavir) and Veklury (remdesivir) are
not available, feasible or clinically appropriate, consider one the following alternative therapies. Continue to
Step 5.
Step 4
Paxlovid (nirmatrelvir co-packaged with ritonavir)2,8 within 5 days of symptom onset if patient does not have severe renal impairment (eGFR <30mL/min) OR severe hepatic impairment (Child-Pugh Class C)
- eGFR ≥ 60mL/min: 300 mg nirmatrelvir taken with 100 mg ritonavir twice daily for 5 days
- eGFR ≥ 30mL/min to < 60 mL/min: 150 mg nirmatrelvir taken together with 100 mg ritonavir twice daily for 5 days
- Evaluate concomitant use of CYP3A inducers and medications with high dependency on CYP3A for clearance as these may be contraindicated2,3,8
OR
Veklury (remdesivir)4 200 mg IV x 1 dose on Day 1, 100 mg IV x 1 on Days 23 begun within 7 days of symptom onset
Prescribers must review and comply with the mandatory requirements outlined in the Paxlovid (nirmatrelvir co-packaged with ritonavir) EUA2 or the Veklury Prescribing Information.4
Step 5
Lagevrio (molnupiravir)5, if age 18 or over and not pregnant (if applicable). 800 mg by mouth every 12h for 5 days begun within 5 days of symptom onset.
- Lagevrio (molnupiravir)5 is not authorized for initiation of treatment in patients hospitalized due to COVID-19.
- Lagevrio (molnupiravir) can be administered via nasogastric (NG) or orogastric (OG) tube (12F or larger), refer to instructions within the EUA Fact Sheet, Section 2.3.
- Prescribers must review and comply with the mandatory requirements outlined in the Lagevrio (molnupiravir) EUA.6
COVID-19 convalescent plasma7 with high titers of anti-SARS-CoV-2 antibodies can also be considered for the treatment of COVID-19 in certain patients with immunosuppressive disease or receiving immunosuppressive treatment.
Step 6
Treatment of symptoms, management per NIH1 & CDC6 Guidelines
1NIH COVID-19 Treatment Guidelines Therapeutic Management of Nonhospitalized Adults With COVID-19
2Paxlovid EUA
3NIH's COVID-19 Treatment Guidelines Panel: Ritonavir-Boosted Nirmatrelvir (Paxlovid)
4Veklury (remdesivir) Prescribing Information
5Lagevrio EUA
6CDC Covid-19 Website
7Fact Sheet for Health Care Providers: EUA of COVID-19 Convalescent Plasma for Treatment of COVID-19
8Paxlovid Prescribing Information
Clinical Decision Aid for Pediatric Patients 28 Days of Age and Older
Pediatric patients 28 days of age and older weighing 3 kg to less than 40 kg with mild to moderate COVID-19 and at high risk for progression to severe disease.
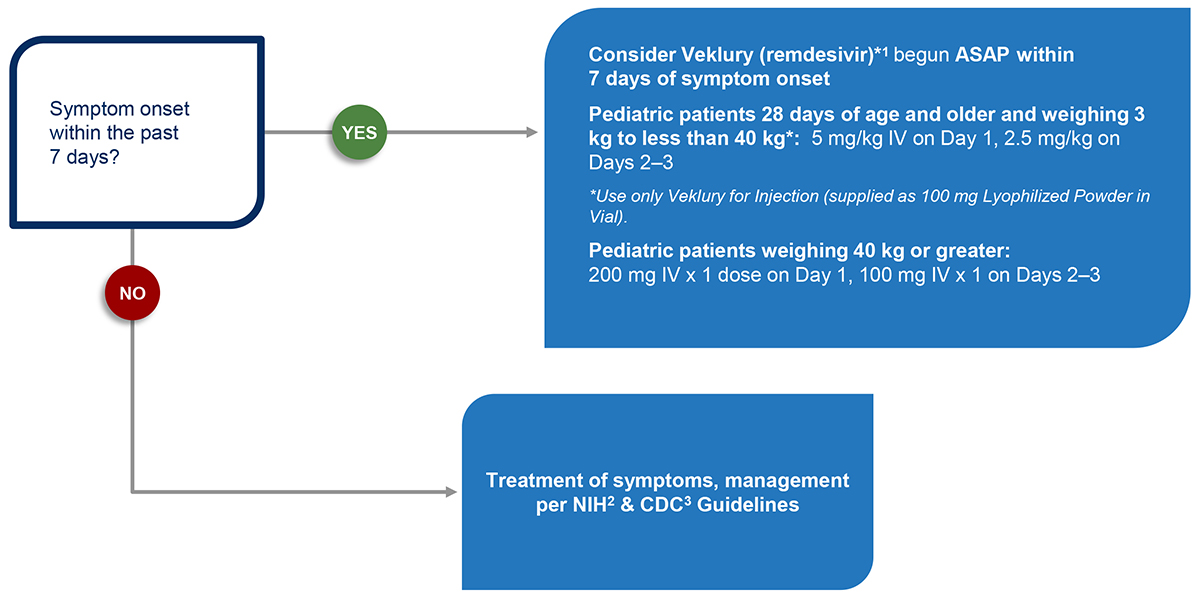
Text Version of Clinical Decision Aid for Pediatric Patients 28 Days of Age and Older Flow Chart
Step 1
Symptom onset within the past 7 days?
Step 2
Consider Veklury (remdesivir)*1 begun ASAP within 7 days of symptom onset.
Pediatric patients 28 days of age and older and weighing 3 kg to less than 40 kg*: 5 mg/kg IV on Day 1, 2.5 mg/kg on Days 2–3
*Use only Veklury for Injection (supplied as 100 mg Lyophilized Powder in Vial).
Pediatric patients weighing 40 kg or greater: 200 mg IV x 1 dose on Day 1, 100 mg IV x 1 on Days 2–3
Step 3
Treatment of symptoms, management per NIH2 & CDC3 Guidelines
1Veklury Prescribing Information.
2NIH COVID-19 Treatment Guidelines Therapeutic Management of Nonhospitalized Adults With COVID-19.
3CDC Covid-19 Website.

