Advancing Biodefense Enterprise Prevention: Goal 2 Biodefense Highlights
Goal 2: Improving Biosafety and Biosecurity to Reduce the Risk of Biological Threats
|
New scientific tools and understanding have created unprecedented opportunities for progress in life sciences research, medicine, and agriculture. Researchers are now capable of using novel scientific technologies and synthetic biology to advance medicine and pharmaceuticals.
|

Figure 3: Trainee at the National Biosafety
and Biocontainment Training Program
|
Synthesis technologies help to advance herapeutic and diagnostic strategies, and the gene-editing system using Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) is a versatile tool, for example, in the search for a Human Immunodeficiency Virus cure and CRISPR-assisted DNA Detection—a promising method for developing diagnostic tests.
Coincident with this era of opportunity, there are elevated concerns about emerging infectious diseases, bioterrorism, and criminal acts involving the misuse of biotechnologies and hazardous biological agents. Laboratory lapses in biosafety and biosecurity could lead to significant public health and economic consequences, particularly if they involve potential pandemic pathogens and/or lack of oversight of dual use research of concern. Preventing the acquisition of equipment, expertise, and pathogenic material for illicit purposes and improving biosafety, biosecurity, and oversight practices in the United States and abroad, are important tenets of our ongoing activities and commitment to national and global health security and international nonproliferation frameworks such as the BWC, United Nations Security Council Resolution (UNSCR) 1540, and the Global Partnership Against the Spread of Weapons and Materials of Mass Destruction.
Biosafety and biosecurity highlights:
Multiple departments and agencies work cooperatively with domestic and international entities, including academia, industry, nongovernmental, professional, and international organizations to strengthen responsible conduct in the life sciences and crowd source the development of training and educational resources on the culture of biosafety and biosecurity. Examples of actions taken by the USG to improve biosafety and biosecurity include implementing federal programs and frameworks and participating in interagency and international collaborations (Table 1); and releasing guidance documents and developing resources for the public.
Table 1: Federal Biosafety and Biosecurity Programs,
Frameworks, and Collaborations
|
Program
|
Department/Agency
|
|
Import Permit Program 4
|
CDC
|
|
Select Agent Program 5
|
CDC, APHIS, FBI
|
|
United States Government Policy for Institutional Oversight of Life Sciences Dual Research of Concern
|
USG-wide
|
|
Department of Health and Human Services Framework for Guiding Funding Decisions about Proposed Research Involving Enhanced Potential Pandemic Pathogens
|
HHS
|
|
Implementation of the biosecurity obligations in UNSCR 1540 6
|
DOS, DOD, DOE, DOJ, HHS
|
Goal 2: Protecting the U.S. Drinking Water Supply against the Risk of Contamination
|
During a naturally occurring, accidental, or deliberate bioincident, unsafe levels of contaminants may be introduced into drinking water. Maintaining a clean supply of water to American citizens during such incidents is therefore a paramount concern. The Environmental Protection Agency (EPA) works closely with federal and SLTT partners to protect drinking water across our country by providing resources to drinking water programs, drinking water systems, and other stakeholders to help them prepare for contamination incidents.
|
 Figure 4: Example of a watermonitoring system
Figure 4: Example of a watermonitoring system
|
Understanding the risk is a necessary first step in preventing and preparing for contamination of the drinking water supply. The
America’s Water Infrastructure Act, signed into law in October 2018, requires that community drinking water systems serving more than 3,300 people conduct a risk assessment and update emergency response plans based on the findings from the risk assessment every five years. The EPA is committed to supporting compliance with this new law by providing training and resources such as the
Baseline Information on Malevolent Acts for Community Water Systems document,
Emergency Response Plan template, and
Vulnerability Self-Assessment Tool.
|
Timely and effective response to contamination requires early detections so that drinking water system operators can implement response plans and take steps to protect public health. Water Quality Surveillance and Response Systems use real time measurements and information from the public to monitor for potential contamination. Afterpiloting the program in five major metropolitan areas, the EPA has developed resources to help communities across the country implement their own Water Quality Surveillance and Response Systems.
|
 Figure 5: Exercising sampling Figure 5: Exercising sampling
procedures that would be used during awater contamination incident.
|
Following detection, laboratory analysis is necessary to confirm the type of contaminant and the amount present. To address this need, the EPA established the Water Laboratory Alliance, a nationwide network of laboratories operating at all levels of government, to quickly support sampling and analysis in response to a suspected contamination incident.
By providing drinking water systems with tools to detect incidents sooner, identify contaminants quicker, and prepare for an effective response, the EPA is enhancing national biodefense capacity by protecting our drinking water from source to tap.
Goal 2: The Antimicrobial Resistance (AMR) Challenge
Since their discovery nearly a century ago, antibiotics have transformed the world, increasing life expectancy and helping to heal serious infections. However, we are now standing on a precipice that could return us to a pre-antibiotic world. Without aggressive action, AMR could limit or possibly reverse our global progress in healthcare, food production, and life expectancy. In 2018, CDC spearheaded the AMR Challenge, one of the most ambitious global initiatives to date to protect people, animals, and the environment from the growing threat of antibiotic resistance.
“Antibiotic resistance will never stop, so we can’t either. The success of the AMR Challenge shows that despite the deadly global threat of antibiotic resistance, public health action and global coordination across every country and industry can keep us a step ahead, even when antibiotics cannot.”
Alex M. Azar II, Secretary,
U.S. Department of Health and Human Services
Given that the AMR threat is multi-sectoral, transboundary, and expanding, it is critical that the United States continues to lead the fight against it and build on the success of the AMR Challenge to combat AMR domestically and abroad.
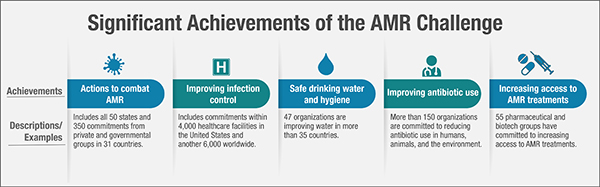
Figure 6: Significant Achievements of the AMR Challenge
Goal 2: Discovery of a New Ebola Species
As part of a project funded by the United States Agency for International Development (USAID) and HHS’s National Institutes of Health (NIH), a new Ebola species, the Bombali ebolavirus, was discovered in healthy bats roosting in people’s homes. The presence of the virus, announced by the Government of Sierra Leone on July 25, 2018, represents the sixth species within the Ebola family and is the first Ebola species discovered before an outbreak in humans.
While no direct evidence exists at this time to suggest the new virus has ever infected humans or livestock, USAID partners have developed communications materials to help communities in Sierra Leone proactively reduce their risk of exposure to Ebola and other viruses originating in bats and other animals. Additionally, for the first time in West Africa, CDC discovered Marburg virus in cave-dwelling bats from multiple sites in Sierra Leone. These discoveries will help Sierra Leone and neighboring countries (1) update their diagnostics to ensure they can detect all Ebola and Marburg viruses, (2) set up early warning human and livestock surveillance, and (3) develop communication materials to help reduce people’s risk of exposure.
<< Previous ---------
Top of Page ---------
Next >>

